New Partial Heart Transplant Program for Semilunar Valve Disease
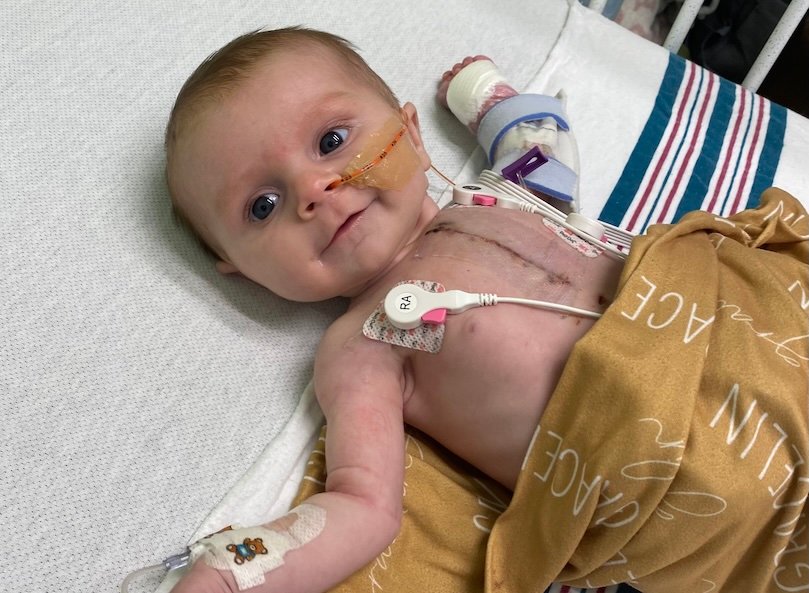
Congenital heart defects involving the semilunar valves (aortic or pulmonary) can increase the workload of the heart and often require early surgical intervention. Children with these kinds of defects exist in an uncomfortable space between less-than-ideal options. A full heart transplant carries a lifelong burden of immunosuppression and limited graft longevity, if they’re able to find a donor heart at all. But standard valve replacements often require repeat surgeries throughout a child’s life.
A new program at UVA Health Children’s treats these defects by transplanting semilunar valves. Unlike traditional bioprosthetic or mechanical valve replacement options, this procedure uses living tissue that retains growth and self-repair potential. It may reduce the need for future surgeries and improve quality of life.
Led by Firezer Haregu, MD, a pediatric cardiologist and researcher, the program places UVA Health Children’s among a small group of institutions exploring this approach. It aims to improve outcomes for children with congenital heart defects while reducing the burden of lifelong immunosuppression.
The Challenges of Pediatric Semilunar Valve Disease
Congenital heart defects involving the semilunar valves can lead to complications such as stenosis, regurgitation, or both. In addition to life-threatening complications, these conditions can make it impossible for children to thrive, leading to developmental delays and other complications that can be halted with early correction.
Historically, pediatric valve replacement options include:
- Homografts (bioprosthetic transplants): Cryopreserved human donor valves that do not grow with the patient and often degrade over time
- Mechanical valves: Durable options that require lifelong anticoagulation and are not suitable for very young children because of size and growth limitations
Both solutions typically require multiple surgeries as the child grows.
Semilunar Valve Transplantation
A partial heart transplant replaces a child’s diseased semilunar valve with a fresh donor valve from a pediatric heart not suitable for full transplant. Unlike cryopreserved valves, these donor valves are not frozen or chemically treated, which helps preserve viable cells and tissue.
Key benefits include:
- Growth potential: The transplanted valve may grow with the patient.
- Self-repair capacity: Viable cells may reduce degeneration and extend valve function.
- Possible reduction in immunosuppression: Early evidence suggests these valves may require less immunotherapy than full heart transplants.
Haregu and the team at UVA Health Children’s aim to offer these valves as a one-time procedure that may reduce the need for future surgeries.
Understanding the Immunologic Advantage
In a recent study published in Pediatric Transplantation, Haregu and his team evaluated semilunar valve function in the context of allograft rejection after full heart transplantation. Findings suggest that these valves may not trigger the same immune response as other heart tissues, which may help reduce or eliminate the need for chronic immunosuppressive therapy.
Key findings include:
- In full heart transplant recipients, semilunar valves appear resistant to immune-related damage, even during rejection episodes.
- Tissue samples from transplant recipients showed preserved valve structure, despite immune cell activity in other parts of the heart.
Haregu notes, “If we can confirm that semilunar valves are truly immune-privileged, this could address one of the most serious risks of transplantation: lifelong immunosuppression.”
Surgical Expertise Supported by Transplant Infrastructure
UVA Health Children’s is the only hospital in Virginia that performs pediatric heart transplants. The nationally recognized pediatric heart transplant team has the experience and infrastructure necessary to help ensure better outcomes for these patients.
Close collaboration between pediatric cardiology, surgery, and transplant care providers means multiple teams with clinical expertise evaluating every patient through a workflow that is tried and true. Patients are also assisted by immunology, social work, and offered financial counseling.
Use of a custom waitlist helps to match children with valve donors, as UVA Health Children’s works on the extraordinary task of improving the matching process for all children awaiting heart transplant.
And in recovery, children have the benefit of a dedicated PICU team with expertise in the needs of children, transplant recipients, and congenital heart disease. Advances like CoMET help clinicians see problems in advance and correct early for better outcomes.
Valve donations may come from:
- Declined full-heart transplant organs
- Domino donations, when children receiving a heart transplant donate their native semilunar valves that remain viable
- Partner organizations that supply harvested valves
Who Benefits Most from This Approach
For children who are stable enough to wait for a matched donor, this procedure offers many benefits.
That includes children with:
- Truncus arteriosus with truncal valve insufficiency
- Tetralogy of Fallot with pulmonary atresia
- Isolated pulmonary or aortic valve dysplasia, stenosis, or regurgitation
For children who aren’t stable enough to wait, UVA Health Children’s cardiologists offer a wide range of procedures, including a Berlin Heart, ECMO, LVADs, and other methods for stabilizing children.
Positioning for Impact
The pediatric cardiology team is committed to improving care for children with congenital heart defects. With clinical expertise, integrated care teams, and established transplant infrastructure, UVA Health Children’s is working to advance the field of semilunar valve transplantation.