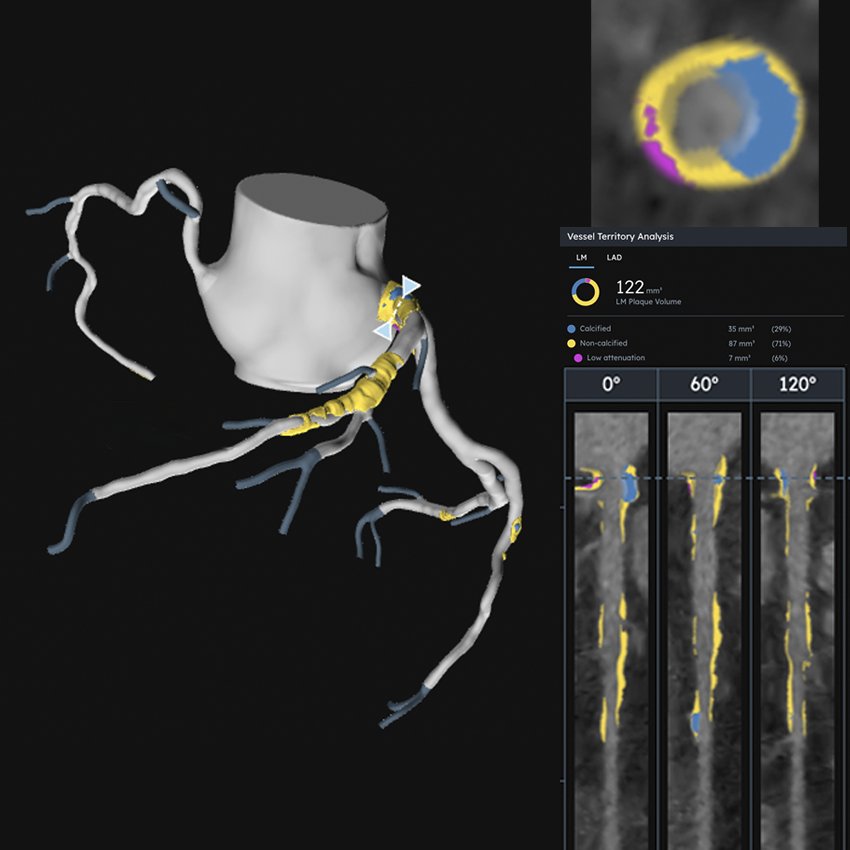
Research Spotlight: Fighting Restenosis With Targeted Nanoparticles
Aiming to uncover new therapeutic targets for heart disease interventions, Lian-Wang Guo, PhD, together with his collaborators, studies how dysregulated chromatin-modulating proteins, which control gene expression in our DNA, may alter vascular cell behavior. By identifying these therapeutic targets and developing lesion-directed delivery strategies, Guo and his team hope to create new treatment opportunities to correct pathological cell states.
See Guo's selected publications. Here, he discusses this research's potential impact on heart disease prevention and targeted care.
Targeted Nanoparticles Could Revolutionize Heart Restenosis Treatment
I do cardiovascular research and I believe it's important. I love what I do. It doesn't feel like a job. It's more like a lifestyle. I enjoy coming to the lab every day, working with young scientists to find something new. My name is Lian-Wang Guo, and I'm a professor in the department of surgery. When an artery is clogged, doctors can insert a balloon catheter to re-open it or use open graft to bypass a clogged artery to resume blood flow. But the problem is the disease will come back, so called restenosis. My lab investigates why it happens and how to prevent it. We are especially interested in some chromatin-associated proteins that regulate disease-related gene programs. Our studies suggest that targeting these epigenetic regulators could outperform current methods by reducing side effects. With collaborators, we have developed nanoparticle systems that can provide localized, targeted, and sustained drug delivery to prevent restenosis. It's so exciting to see the real potential of our work to help patients. This kind of innovation supports the mission of the Manning Institute of Biotechnology.
What are you working on right now?
I study how dysregulated chromatin-modulating proteins drive vascular disease, and how we can target them with precision to improve therapeutic safety and efficacy.
When the blood vessel wall becomes too thick, as in atherosclerosis and restenosis, blood flow is disrupted. When the wall becomes too weak, as in aneurysms, it can rupture. In vital vessels, either outcome can be life-threatening.
Vascular health depends on the proper function of the cells in the vessel wall, which is largely governed by chromatin, the dynamic architecture that organizes DNA in the nucleus. While DNA stores genetic information, chromatin-modulating proteins determine which genes are turned on or off. These epigenetic “writers,” “readers,” and “erasers” add, interpret, and remove chemical marks on chromatin to control gene expression. Our lab studies how abnormalities in these regulators disrupt normal vascular cell function and drive disease, and we're developing targeted strategies to translate the mechanistic insights into potential therapies.
What are the most intriguing potential clinical applications of your work?
One promising direction is the development of medicines that act on chromatin-modulating proteins. For many years, these proteins were considered “undruggable,” but new technologies, including AI, are helping turn them into therapeutic targets. Because chromatin marks are reversible, targeting these pathways may provide effective and safer treatments without permanently altering a person’s DNA.
Another major challenge in medicine is delivering therapies only where they're needed while minimizing side effects on the rest of the body. Working with collaborators across disciplines, we're innovating delivery approaches for localized treatment, including tissue-adhesive nanoparticles and biomembrane-camouflaged, lesion-homing nanoplatforms. Our goal is to achieve dual precision: mechanistic precision through selective molecular targeting and delivery precision through localized therapeutics, ultimately improving safety, efficacy, and tolerability.
What made you choose UVA Health as the place to do your research?
UVA Health is an outstanding environment for research that bridges basic science and patient care. Here, I'm able to collaborate with leading experts in engineering, nanotechnology, genomics, physiology, and surgery, and I have access to programs designed to help move discoveries from the laboratory toward real-world clinical application.
This collaborative and translational ecosystem makes UVA Health an ideal home for our research.
What do you wish more people knew about your area of research?
Vascular disease often develops gradually over many years. By reshaping the landscape of chromatin marks, lifestyle and environmental factors, including diet, physical activity, smoking, and exposure to harmful substances, can profoundly influence gene expression. Over time, these molecular changes can shift vascular cells toward disease.
The good news is that healthy habits truly matter at the molecular level and can help protect blood vessel health.
How did you become interested in your area of research?
Earlier in my career, I focused on basic science, studying protein–protein interactions in test tubes. Later, an opportunity arose to collaborate with surgeons and engineers on preclinical studies, and I began testing strategies to correct dysregulated chromatin modulators in living disease models. Seeing our work move closer to real-world clinical use was deeply motivating and gratifying. Because vascular disease affects so many patients and families, I feel a strong responsibility to pursue research that can ultimately improve patient care.